US FDA issues health warnings on microneedling devices and makeup remover wipes
Key takeaways
- The US FDA has issued warnings about radiofrequency microneedling devices after reports of burns, fat loss, scarring, and nerve damage from dermatologic procedures.
- The agency emphasized that microneedling is a medical rather than cosmetic procedure and is unsuitable for home use.
- Kenvue has voluntarily recalled makeup remover from Neutrogena Makeup due to bacterial contamination concerns.
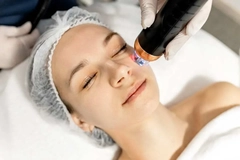
The US Food and Drug Administration (FDA) has issued warnings and a class two health risk on beauty appliances and personal care products this week amid reported health concerns.
It has taken action against radiofrequency (RF) microneedling devices following reports from consumers experiencing burns, fat loss, disfigurement, scarring, and nerve damage from aesthetic and dermatology procedures.
Meanwhile, Kenvue has reportedly voluntarily recalled its Neutrogena makeup remover wipes after bacterial contamination led the FDA to classify the products as a Class II health risk.
The class two health risk refers to a situation “in which use of, or exposure to, a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote,” says the FDA.

Microneedling worries
The FDA issued its warning on RF microneedling devices on October 15 to raise awareness of complaints reported from using the devices. The tools are designed to improve skin appearance and treat wrinkles, thereby tightening and “rejuvenating” the skin.
However, the FDA received multiple reports of serious complications requiring medical or surgical intervention.
“While the FDA’s evaluation is ongoing, we are asking patients, caregivers, and health care providers to report any complications related to using these devices for dermatologic or aesthetic skin procedures to the FDA. Prompt reporting of adverse events can help the FDA identify and better understand the risks associated with medical devices,” the FDA stated.
The regulator urges the public to understand that microneedling is a medical procedure, not a cosmetic treatment. “These devices should not be used at home,” it says.
Neutrogena’s wipes
The parent brand of Neutrogena, Kenvue, issued a voluntary recall in four US states: Texas, South Caroline, Georgia, and Florida. The recall concerns Neutrogena Makeup Remover Ultra-Soft Cleansing Towelettes, after the products were found to be contaminated with Pluralibacter gergoviae bacteria.
The recall was issued following an internal investigation, which found the bacteria were present in specific product batches.
The contamination poses serious health risks, especially for individuals with compromised immune systems. It can cause respiratory issues, urinary tract infections, and kidney problems.
Exposing the eye area to this bacteria could also lead to infections, vision problems, or permanent damage.
At the time of publication, Kenvue has not released an official statement about the reported recall.













