US FDA warns of fake Botox sold online due to “serious health risks”
Key takeaways
- The US FDA has issued warning letters to 18 websites selling unapproved or counterfeit Botox products.
- Fake botulinum toxin injections can cause botulism symptoms, which are life-threatening.
- The FDA urges patients to receive Botox treatments only from licensed providers using approved products.
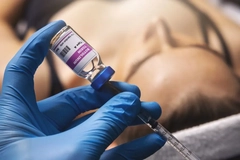
The US Food and Drug Administration (FDA) has issued warning letters to 18 website owners illegally marketing unapproved and misbranded botulinum toxin products, commonly referred to as Botox.
The agency has received adverse event reports associated with these types of botulinum toxin products, including botulism symptoms. Botulism attacks the body’s nerves and causes muscle weakness that can lead to difficulty breathing and death. The US FDA advises seeking immediate medical care if any symptoms occur.
“Unapproved and misbranded Botox products carry serious health risks. Today we’re taking action to protect American consumers and prevent online entities from selling these dangerous products,” says FDA Commissioner Marty Makary.
In 2022, over 9 million Botox treatments were administered globally, representing a 26.1% increase from the previous year, according to Spa Medica.

According to the American Society of Plastic Surgeons, between 2019 and 2022, the number of people 19 and younger receiving Botox-style injections increased by 75%. Use also rose 71% in adults 20 to 29.
Botulinum toxin dangers
The FDA said the warned companies were offering unofficial or mislabeled versions of Botox-like drugs that the agency has not approved. Some of the websites named include maypharm.net, cosmo-korea.com, and aesthetic-essentials.com.
There are several FDA-approved botulinum toxin products available on the market, such as Botox, which are only available with a prescription from a licensed health care professional.
Botox is a diluted, purified form of botulinum — one of the most toxic substances in the world.
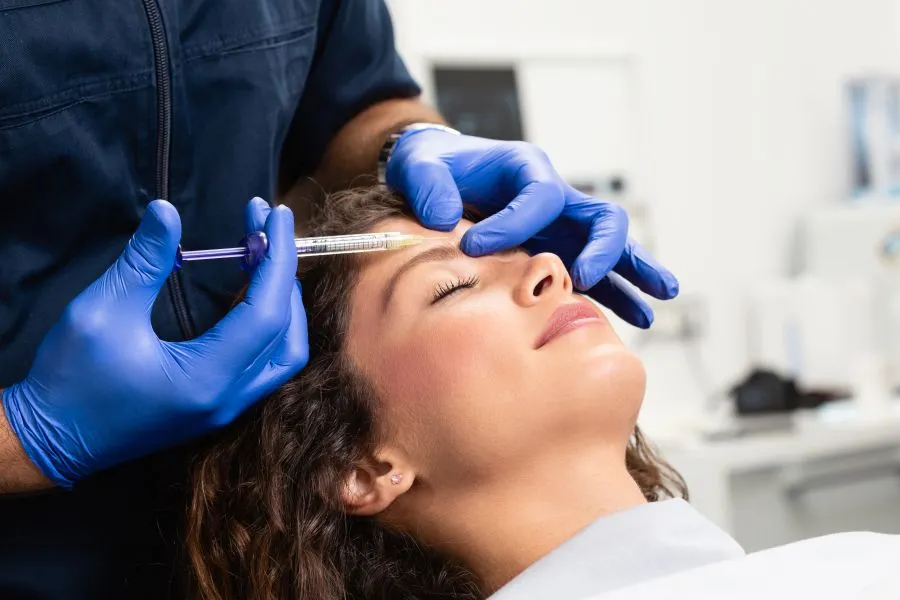 Counterfeit Botox poses health risks.Botulinum toxin products are injectable drugs that block nerve activity in muscles, temporarily reducing muscle movement. They are most known for their cosmetic use, such as reducing facial wrinkles. However, they also treat medical conditions such as chronic migraines and muscle stiffness.
Counterfeit Botox poses health risks.Botulinum toxin products are injectable drugs that block nerve activity in muscles, temporarily reducing muscle movement. They are most known for their cosmetic use, such as reducing facial wrinkles. However, they also treat medical conditions such as chronic migraines and muscle stiffness.
The US FDA-approved botulinum toxin products carry a boxed warning — the agency’s most serious warning — indicating the drug carries a significant risk of serious or life-threatening side effects.
The boxed warning indicates the product may cause symptoms of botulism. In rare cases, the toxin can spread beyond the injection site to other parts of the body, paralyzing or weakening muscles needed for breathing and swallowing.
The FDA urges patients only to receive Botox-type injections if they are obtained from an authorized source and administered by a licensed and trained provider.
“Products purchased from unauthorized sources may be unapproved, misbranded, adulterated, counterfeit, contaminated, improperly stored and transported, ineffective, and/or unsafe,” says the agency.
Health care professionals and consumers should report adverse events to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
FDA adverse event warnings
The US FDA recently launched a public dashboard for consumers to access and report cosmetics that it believes have exhibited adverse effects. The interactive tool aims to increase government–constituent transparency.
US FDA opens public portal for reporting cosmetic adverse events
In other cosmeceutical warning announcements, the FDA cracked down on radiofrequency microneedling devices following reports from consumers experiencing burns, fat loss, disfigurement, scarring, and nerve damage from aesthetic and dermatology procedures.
Additionally, following adverse event reports, it issued a renewed warning about temporary tattoos and “black henna” body art.