US and UK look to cosmetics industry to end animal testing
Key takeaways
- The US and UK are both moving toward wider use of non-animal test methods, with cosmetics playing a central role in the shift.
- PCPC and PETA welcome the passage of a US bill supporting modern sunscreen evaluation and prioritizing NAMs.
- The UK’s new five-year strategy to phase out animal testing uses the cosmetics sector as a working model.
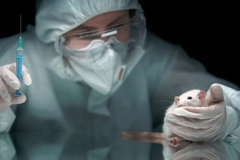
Growing pressure to modernize cosmetics safety testing away from animal use is shaping regulatory shifts on both sides of the Atlantic. The UK has launched a national strategy to phase out animal testing, citing the cosmetics sector as a successful early adopter of New Approach Methodologies (NAMs). Meanwhile, in the US, the Continuing Appropriations Act has created renewed attention on non-animal test methods for cosmetic ingredients.
The Personal Care Products Council (PCPC) views the US bill as an important step toward supporting cosmetics innovation and protecting public health. The group says the US FDA now has stronger funding, which can support progress toward more modern, cruelty-free safety approaches.
PETA also welcomes the US bill and its implications for cruelty-free science. The animal-rights group says the FDA should prioritize using NAMs to support wider access to modern UV filters.
US market impact
The PCPC welcomes the Continuing Appropriations Act (H.R. 5371) as a positive step for the personal care industry, especially regarding the full-year funding measures for the US FDA.
“This action ensures that the agency has the necessary resources to safeguard public health and foster innovation,” the group says in a statement.
The PCPC links the bill to progress in cruelty-free safety testing. In its statement, the organization says the move represents “meaningful progress toward the regulatory acceptance of non-animal test methods.”
This aligns with shifts already visible in global cosmetics safety assessments, where in vitro and computational tools are becoming more common.
Moreover, the group emphasizes the need for continued investment in modernizing cosmetic safety. The PCPC states that it is “pleased that H.R. 5371 also includes dedicated funding for the FDA to implement key provisions of the Modernization of Cosmetics Regulation Act of 2022.”
The organization says the funding will enable the FDA to continue developing its new systems for facility registration, product listing, and safety checks. It will also support the agency as it works on good manufacturing practices and updated rules for fragrance allergen labeling.
The bill includes measures that the PCPC says will help expedite FDA processes. One such measure is the reauthorization of the Over-the-Counter Monograph User Fee (OMUFA) program through 2030.
.webp) Advanced in vitro systems offer human-relevant insights without relying on animals.“The OMUFA reauthorization includes critical language enabling the FDA to consider broader evidence when evaluating the safety and efficacy of topical products such as sunscreen. This is a significant step in promoting skin cancer prevention by ensuring US residents have access to innovative and effective sunscreen ingredients,” says the PCPC.
Advanced in vitro systems offer human-relevant insights without relying on animals.“The OMUFA reauthorization includes critical language enabling the FDA to consider broader evidence when evaluating the safety and efficacy of topical products such as sunscreen. This is a significant step in promoting skin cancer prevention by ensuring US residents have access to innovative and effective sunscreen ingredients,” says the PCPC.
According to the organization, the bill could play a key role in bringing new UV filters to the US market.
The support reflects long-standing concerns from companies about the slow pace of sunscreen approvals in the US, compared to regions such as the EU and Asia. The FDA has not approved a new UV filter since 1999.
Animal-free call
PETA responded to the bill from a different angle but expressed similar optimism.
The animal-rights group says the bill “shows congress agrees with PETA and tens of thousands of PETA supporters: the FDA must prioritize non-animal test methods for evaluating sunscreens and must expand access to modern, effective sunscreens to enhance protection against skin cancer.”
PETA emphasizes that scientific work on new test approaches has been underway for decades.
“We have sent multiple letters to the agency, urging it to act swiftly by collaborating with sunscreen manufacturers and other scientific experts who have spent decades developing reliable cell- and computer-based test methods to assess sunscreen safety,” the organization says.
PETA argues that these modern NAM systems offer more human-relevant insights than legacy animal studies. The group also notes that the bill creates an opportunity for the FDA to work closely with companies that are already using such methods.
According to PETA, “congress has made it clear that it expects the FDA to find ways to accelerate the use of non-animal tests and to give consumers confidence that the sunscreens they use are safe.”
The group further describes the bill as “an opportunity for the FDA to engage with the many companies that are interested in using more innovative and human-relevant non-animal testing methods.”
.webp) Modern NAM tools such as organoids and 3D skin models are reshaping cosmetics safety testing.Cosmetics’ UK template
Modern NAM tools such as organoids and 3D skin models are reshaping cosmetics safety testing.Cosmetics’ UK template
Across the pond, the UK government’s new five-year strategy outlines how it plans to phase out animal testing “in all but exceptional circumstances.” The strategy introduces clear timelines, funding commitments, and new regulatory structures to accelerate the shift toward modern NAMs.
Cosmetics testing on animals has been banned in the UK for decades. Thereby, the strategy highlights the cosmetics sector as a working example of how validated NAMs can replace animal tests while still meeting regulatory standards.
Regulators cite in vitro skin and eye irritation assessments as evidence that human-relevant methods can be applied at scale.
Cruelty Free International (CFI) welcomes the announcement, calling it “a long overdue but very exciting move toward ending the cruelty of animal testing in the UK.”
The organization says the plan marks “a strong step forward” on the government’s 2024 manifesto commitment to phase out the use of animals in science. Moreover, CFI claims the strategy demonstrates that sectors like cosmetics have already paved the way for wider adoption of alternative methods.
The government strategy includes several time-bound targets to eliminate specific tests where validated alternatives already exist.
According to CFI, the government has committed to “fully replace the rabbit pyrogen test by the end of 2025,” and to “end skin and eye irritation and corrosion testing on animals by the end of 2026.”
It also plans to fully replace skin sensitization testing on animals by the end of 2026 and end Botox potency tests on mice by 2027. CFI notes that these tests appear on its Replace Animal Tests list, where non-animal approaches are already available.
Infrastructure and regulatory reform make up another part of the plan. The government will invest £30 million (US$39.4 million) in a preclinical translational models hub by next year. It will also establish a UK Center for the Validation of Alternative Methods to streamline the path from new method development to regulatory acceptance.
CFI says these commitments are essential, adding that “the commitments to funding, regulatory reform, and measurable targets are exactly the kind of leadership we need.”













